
In addition to pharmaceuticals, toxicity can also occur from exposure to a number of plants and animals that contain cardioactive corticosteroids, including dogbane, foxglove, lily of the valley, oleander, yellow oleander, red quill, and the Bufo species toad. Description: Digoxin immune fab is an antidote for digoxin toxicity. Serum digoxin concentration is usually greater than the therapeutic range of 0.5 to 0.9 nanograms/mL, but may not be elevated. Manifestations of life-threatening toxicity of digoxin overdose such as severe ventricular arrhythmias, progressive bradycardia, and second or third degree heart block not responsive to atropine, serum potassium levels exceeding 5.5 mEq/L in adults or 6 mEq/L in children with rapidly progressive signs and symptoms of digoxin toxicity. For digoxin tablets, calculate number of antidote vials as follows.

In therapeutic doses, digoxin increases cardiac contractility and controls the heart rate.ĭigoxin toxicity is a clinical diagnosis that relies in part on ECG findings such as signs of increased automaticity and atrioventricular node blockade (premature ventricular contractions, slowed ventricular response). di-JOX-in DigiFab Therapeutic class: Antidotes Pharmacologic class: Antibody. An account of the foxglove, and some of its medical uses: with practical remarks on dropsy and other diseases. It is indicated for the treatment of mild to moderate heart failure. 26 It is very similar to Digibind, except that it is prepared using a digoxin derivative (digoxin-dicarboxymethoxylamine) as the hapten.Digoxin is the most commonly prescribed cardioactive corticosteroid in the US. 25 Another commercial product, DigiFab, approved by the US Food and Drug Administration (FDA) in 2001, is currently available. Call your doctor for medical advice about serious side effects or adverse reactions. This is not a complete list of side effects and other serious side effects or health problems that may occur as a result of the use of this drug.

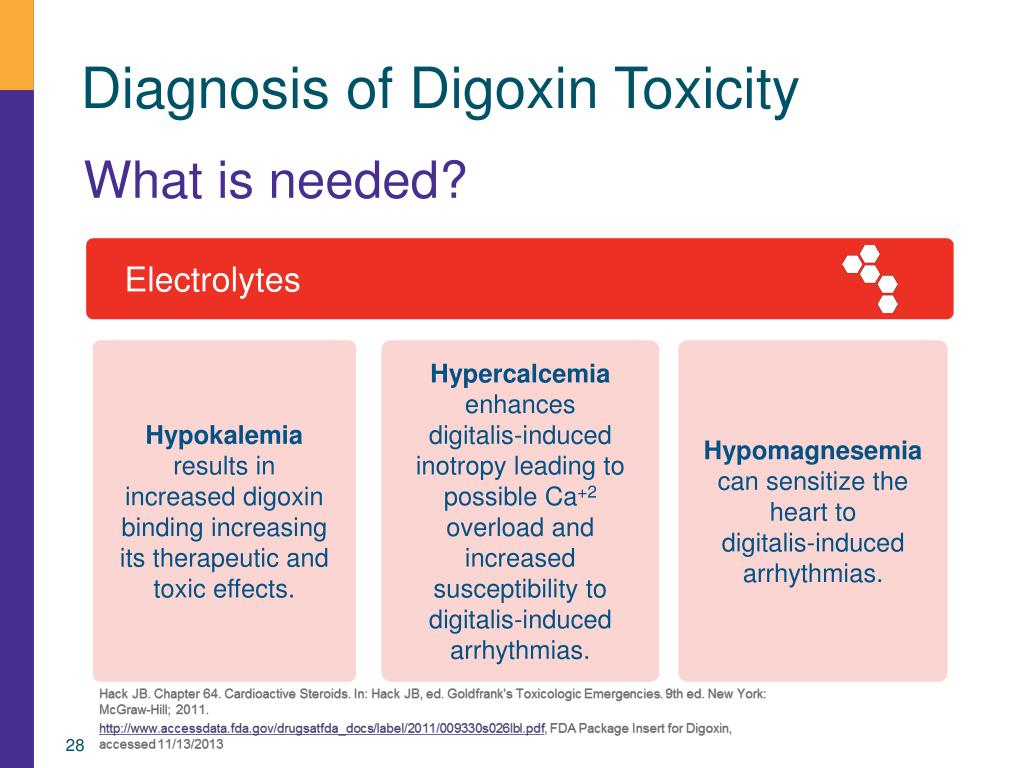
Rare side effects of Digoxin Immune FAB include: none. 14, 48, 51, 53 In 1976, Digibind was used with clinical success, 81 and it became commercially available in 1986 before being discontinued in 2011. numbness or tingling, muscle weakness, and. When compared with whole IgG antibodies, the advantages of DSFab included a larger volume of distribution (Vd), more rapid onset of action, diminished risk of adverse immunologic effects, and more rapid elimination. Since the Fc fragment does not bind antigen and increases the potential for hypersensitivity reactions, it was eliminated. 14 Affinity chromatography is used to isolate and purify the DSFab following papain digestion. At therapeutic digoxin doses (0.5 to 0.9 nanograms/mL), the ECG typically shows PR-interval prolongation and a scooped ST segment. Diagnosis is based on symptoms and laboratory data. To make such antibodies both safe and effective in humans, whole IgG antidigoxin antibodies were cleaved with papain, yielding two antigen-binding fragments (Fab), with a molecular weight of 50,000 Da each, and one Fc fragment of 50,000 Da. Typically presents with components of gastrointestinal, constitutional, and/or cardiovascular symptoms. Furthermore, there was significant concern with regard to the development of hypersensitivity reactions.
#Digoxin antidote free
14 Unfortunately, the urinary excretion of digoxin was delayed, and free digoxin was released after antibody degradation occurred. Intact IgG antidigoxin antibodies reversed digoxin toxicity in dogs.


 0 kommentar(er)
0 kommentar(er)
